One of the main causes for high failure rates of clinical trials is the suboptimal definition of patient selection criteria. Researchers from Arcturis demonstrate how real-world evidence can inform and optimise patient selection criteria on the THEMIS trial. By modifying the enrolment criteria, the size of the eligible patient population and required patient numbers can be improved, thus reducing recruitment costs and increasing the overall success rate of the trial. Furthermore, real-world data can help to investigate the impact of selection criteria on patient diversity.
THEMIS(1) – A multinational, randomised, double-blind, placebo-controlled phase IIIb trial run by AstraZeneca in 2014 evaluated the effect of ticagrelor twice daily on the incidence of cardiovascular death, myocardial infarction, or stroke in patients with type 2 diabetes mellitus. This study enrolled 19,271 patients from 1,315 sites from 2014 to 2019.
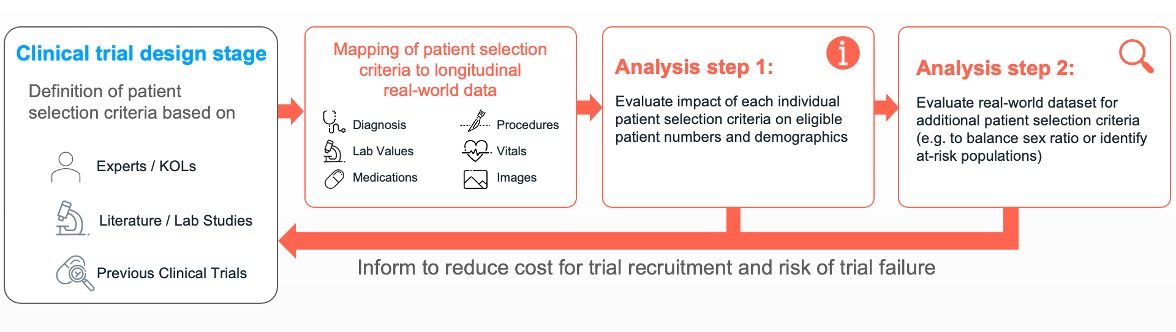
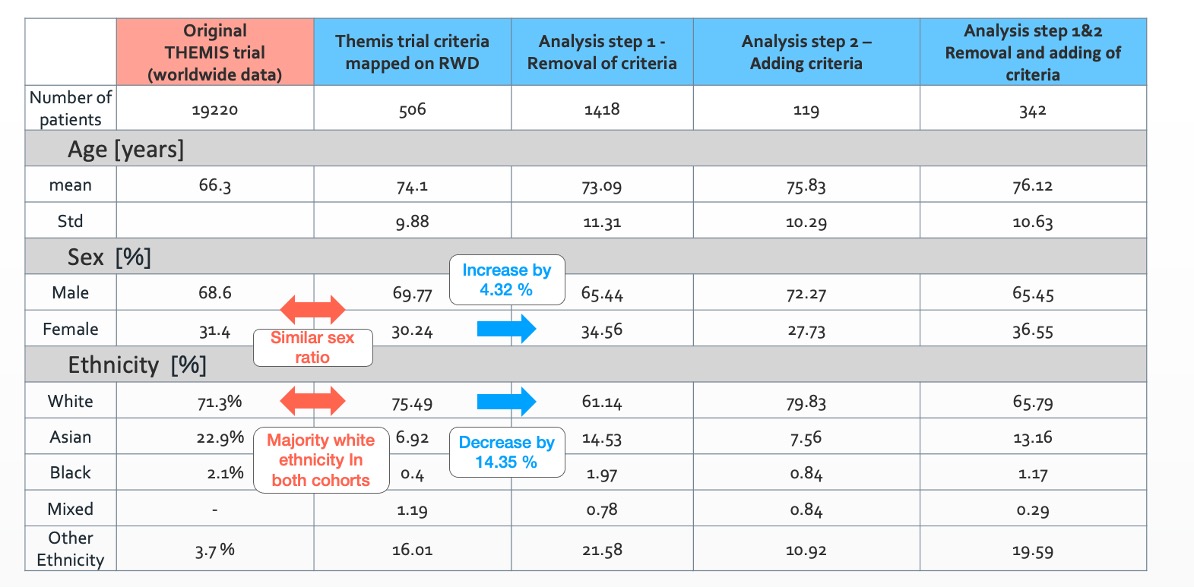
Arcturis collected a dataset consisting of over 1million patients with a wide range of cardiovascular diseases from real-world clinical settings with our NHS trusts partners. In a first step, we mapped the inclusion and exclusion criteria of the THEMIS trial to real-word clinical observations. Due to the richness and longitudinal nature of our real-world data (RWD), 17 of the 19 patient selection criteria could directly be translated into real-world practice. The resulting cohort of 508 patients was evaluated with respect to mortality as the primary study outcome. We observed a similar sex and ethnicity ratio in this UK-based cohort compared to the THEMIS trial.
In the first analysis step, data scientists and clinicians at Arcturis evaluated the impact of individual patient selection criteria on the size and the diversity of the cohort with respect to etchnicity, age, and gender. By removal of the following 3 criteria:
- Inclusion criteria: ≥50 years of age,
- Inclusion criteria: Prescribed glucose lowering medication for least 6 months,
- Exclusion criteria: Patients with kidney failure requiring dialysis,
the number of eligible patients could be increased by a factor of ~3 from 508 to 1418 patients. Additionally, the ratio male/female became more balanced and the percentage of white people within the cohort dropped from 75.49% to 61.14%. These insights can be used to define diversity plans as suggested by FDA(2) to improve trial diversity.
Demographic comparison of original recruited cohort for the THEMIS trial and for the different cohorts investigated using real world data.
In a second analysis step, we evaluated if there are clinically meaningful criteria that could be added to reduce the number of required study participants. As outlined in the enrichment strategies for clinical trial by the FDA guidelines(3), one option to reduce the number of required patients is by focusing on a patient sub-cohort with an increased risk profile. We applied a machine learning algorithm on all clinical observations available in our RWD to identify potential additional patient selection criteria. After clinical evaluation, 2 criteria were investigated further:
- Previous record of heart failure, and
- Previous record of atrial fibrillation and flutter.
Adding these criteria resulted in an increase of the baseline event rate from 0.16 [events/patient-year] to 0.31. Performing a power analysis (assuming the same treatment effect), the number of required patients could be reduced by 37.9%, which would significantly reduce recruitment costs. Note, this process should be support ideally by clinical experts which understand the mode of action of the drug to better evaluate which criteria can be removed or added, which was not available for this project.
In summary, we presented an approach how real-world evidence can help to optimise clinical trial criteria to improve patient diversity and reduce the number of required trial participants. This process can be used, for instance, at the clinical trial design stage to inform about the impact of already existing criteria and to suggest additional criteria to reduce recruitment costs and increase the probability of success of the overall trial.